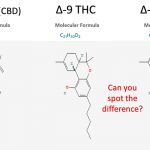
Tetrahydrocannabinol (THC) is the most well known psychoactive molecule found in the inflorescence (bud) of Cannabis sativa. Numerous natural analogs of THC found in Cannabis perform similar effects when ingested by consumers. These species sharing a similar scaffold have a slight difference in chemical structure differentiating them from Δ9-THC. Δ8-THC…